In the spotlight
Prime-time Enzymes
Need to know
Enzymes. Ubiquitous and yet, unavoidably destined to be starlight influencers, these little proteins will change products and industries, shape wastewater treatment, and reduce energy consumption. But watch out: there’s more to come from the cold-hearted ones.
We all know it: the scarcity of drinking water is the single most pressing problem humanity has to solve. A challenge brought about by climate change, growing population, and rapid industrialization. The latter comes on board not only because of the water footprint in every product or service we consume but also due to water contamination in the manufacturing process. Pollutants from industrial activities contaminate the water supply and threaten local and worldwide environments. Commonly known pollutants include industrial dyes, polycyclic aromatic hydrocarbons, chlorinated organic compounds, and dioxins. Other than industries, agricultural and daily household chores also contaminate the water supply and generate wastewater. The dumping of pesticides and sewage waste into water sources is a major problem.
Of course, various physical and chemical technologies have been developed to treat wastewater, such as coagulation, flocculation, activated carbon adsorption, and sanitizers such as chlorine.
There is also great hope for more advanced technologies, including reverse osmosis, nanofiltration, photolysis, ion exchange, and advanced oxidation. However, the cost of these treatments for urban wastewater is prohibitive for many states and nations, and a more affordable solution has to be found.
Biological technologies
In recent years, biological, enzyme-based methods for wastewater treatment have received prime-time attention. They are important because they can completely oxidize many toxic impurities and can be reusable while requiring comparatively low cost and simple equipment.
Enzymes are proteins specialized in the catalysis of biological reactions, and their catalytic efficiency is hundreds or thousands of times higher than that of inorganic catalysts. In addition to the characteristics of general chemical catalysts, enzymes have the advantages of high catalytic efficiency, strong specificity, and mild reaction conditions.
There are different types of enzymes, even in the human body, but those we care about for water treatment are produced by a large number of microorganisms and plants.
These enzymes treat wastewater by breaking down and transforming harmful contaminants into benign, easily collected, easily cleaned biodegradable parts. The treatment eliminates or reduces water toxicity and makes the water supply fit for reuse. Moreover, the enzyme-catalyzed transformation product exhibits minimal toxicity and is more readily biodegradable than the parent compound.

Enzymes for wastewater treatment can be introduced alone or mixed with microbes in the water source. They can also be deployed by introducing whole plants or their tissues that contain large amounts of enzymes in their natural form.
Natural, biodegradable, non-toxic, low-cost. So, where’s the catch?
Enzymes have great specificity, which means that specific enzymes are needed to catalyze specific pollutants in specific concentrations, which calls for a new field of study called Enzyme engineering, which is the part of Biotechnology formed by the combination of enzyme theory and chemical technology.
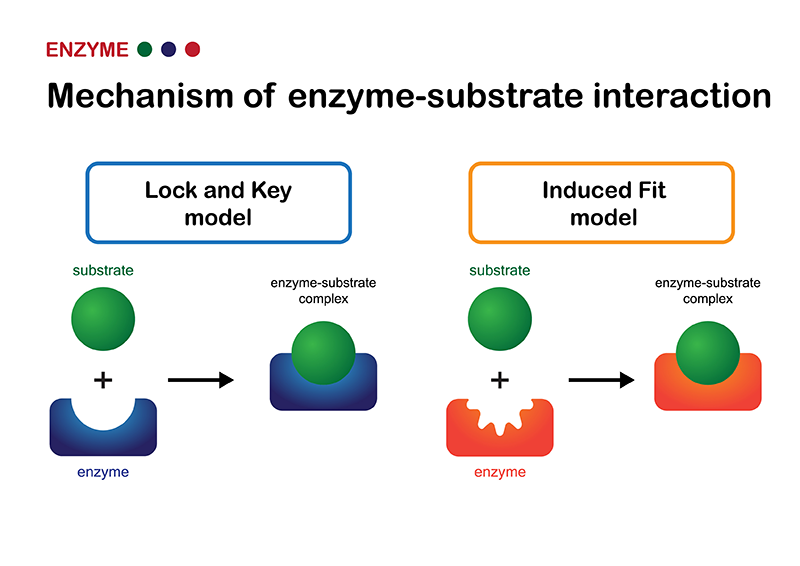
Fitting the key
It was first thought that enzymes interacted with the substrate under a lock-and-key model, that is, a perfect fit. But today it is widely recognized that they adapt to achieve that fit into what is called an induced fit model.
Enzymes are used in the pharmaceutical industry, the food industry, and the light industries, such as feed, textile, papermaking, leather, and detergent, among others.
Customized enzyme products are prepared to solve particular issues. For example, the textile dyeing process generates wastewater, where we find organic compounds such as phenols, chlorinated phenols, aromatic compounds, and certain colorants.
This type of compound makes their removal difficult with conventional wastewater treatment methods since most systems based on physical-chemical treatments are expensive and require a large amount of energy and agents.
But peroxidase and lactase enzymes can do part of the job pretty well.
Peroxidases are present in all life forms, from decomposers, and producers to consumers. They prevent oxidative damage in plant leaves and act in the lignification process. They mediate the reduction of peroxides, especially hydrogen peroxide (H2O2) or any organic peroxide, accompanied by the oxidation of chemically diverse compounds. Peroxidase activity precisely entails the transfer of an electron to the substrates, causing them to be broken down into harmless components in the reaction. The peroxidase reaction mainly includes four kinds, oxidative dehydrogenation, oxidative halogenation, H2O2 disproportionation, and oxygen transfer reaction. These four reactions have peroxidase centrality in the treatment of recalcitrant heterogeneous wastewater contaminants such as dyes, phenols, and aromatics from domestic or industries.
The second part of the job lies in the hands of microorganisms, which are capable of degrading and eliminating the color of wastewater through different mechanisms, such as bioabsorption, aerobic or anaerobic biodegradation, and production.
We should bear in mind that the production of the clothes we wear every day involves the emission of chemical substances that are equivalent to the emissions of a car driving almost 100 km. It also involves the consumption of up to 15,000 liters of water, and an energy expenditure equivalent to half a year of a home. The use of enzyme solutions for textile bioprocessing may reduce toxic emissions by up to 75%, and energy, water, and chemical consumption by 40-70%.
Enzymes are also transforming detergents, where they help eliminate dirtiness and grease without the use of phosphates, a component historically used that causes extreme environmental damage and is thereby forbidden in the UE and the US.
Extremozymes, the new hero-zymes
In this exciting upsurge of enzyme technology, one of the most intriguing areas is that of extremozymes.
Extremophilic microorganisms have developed unique molecular means to cope with extreme temperatures, acidic and basic pH, high salinity, high radiation, low water activity, and high metal concentrations among other environmental conditions. Extremophile-derived enzymes, or extremozymes, might well enclose a hidden treasure, as they may be able to catalyze chemical reactions under harsh industrial processes.
The laundry and dishwashing industry is actively looking for cold-active enzymes to do the job under the current cold-washing practices, which can work optimally at low temperatures (15–25°C), but maintain a high activity through a wide range of temperatures (between 5 and 60°C), and stay active in the presence of surfactants and alkaline pH are the future for this industry. The outcome of these adaptable enzymes’ use would be a reduction in laundry energy and water utilization.
Every watt counts. Every drop of water. Every enzyme we can ferret out.

